Massachusetts: Lecanemab, the drug which is designed to target and clear amyloid, has become the first drug to slow the destruction of the brain in Alzheimer’s disease.
The new drug has been hailed as a major breakthrough in Alzheimer’s treatment, soon after it was found to reduce memory loss in patients in the early stages of the disease.
Lecanemab is a synthetic antibody, which is similar to those the body produces to fight germs or viruses, that stimulates the immune system to remove amyloid from the brain.
According to Professor John Hardy, group leader at the UK Dementia Research Institute at University College London, this trial is an important first step, and it represents the “beginning of the end.”
The first step is the hardest, and we now know exactly what we need to do to develop effective drugs. It’s exciting to think that future work will build on this, and we will soon have life-changing treatments to tackle this disease.
Prof. John Hardy remarked.
The results of the phase three clinical study have been released by Eisai, a Tokyo-based pharmaceutical company that collaborates with the US biotech company Biogen to manufacture Lecanemab. In September 2022, Eisai released the preliminary findings of a study including 1,795 participants with early-stage Alzheimer’s disease.
The complete study has been published in The New England Journal of Medicine, with experts claiming it as long-awaited evidence that Alzheimer’s disease can be treated.
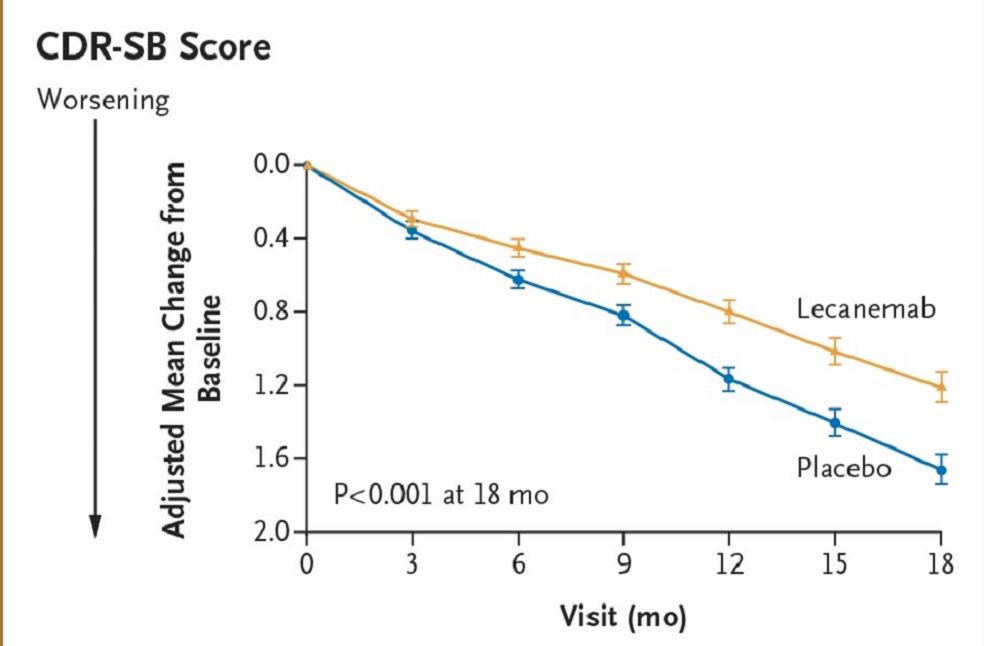
Lecanemab reduced markers of amyloid in early Alzheimer’s disease and resulted in moderately less decline on measures of cognition and function than placebo at 18 months.

Prof. Bart De Strooper, Molecular Biologist at the Vlaams Instituut voor Biotechnologie, stated that, “the overall conclusion is extremely positive. This trial proves that Alzheimer’s disease can be treated.”
Currently, certain medications are given to Alzheimer’s patients to help manage their symptoms, but none change the course of the illness.



